Ecological Interaction between Submerged Macrophytes and Zoobenthos
Corresponding Author
Tel/Fax: +86-21-65980763, E-mail: shpcheng@tongji.edu.cn; Address: 1239 Siping Road, Shanghai 200092, China
Affiliation
Siu Yeon Tan, Zhu Li, Shuiping Cheng*
1 Tongji University, Key Laboratory of Yangtze River Water Environment, Ministry of
Education, Shanghai 200092, PR China
2 Hubei University of Arts and Science, Institute of Hanjiang, Xiangyang 441053, PR China
Article Reviewed By:
James Wong(tfwongah@connect.ust.hk)
Jarno Turunen(Jarno.Turunen@ymparisto.fi)
Dr. Enrique Torres(torres@udc.es)
Shuangshuang Li(lishuangs2010@163.com)
Citation
Siu Yeon Tan, Zhu Li, Shuiping Cheng, Ecological Interaction between Submerged Macrophytes and Zoobenthos(2017)SDRP Journal of Earth Sciences & Environmental Studies 2(2)
Abstract
Aquatic ecosystems are highly influenced by the interaction between submerged macrophytes and zoobenthos within the water column and the sediment underneath. This review paper discusses the complicated bilateral relationship within these components by describing the links and how submerged macrophytes drive the zoobenthos and vice versa. Submerged macrophytes provide food, habitat and nutrients to zoobenthos while zoobenthos provide services such as decomposing plant debris, enhancing materials exchange through bioturbation and providing carbon dioxide for photosynthesis. The interaction mechanisms between submerged macrophytes and zoobenthos can be divided into a bottom-up and top-down effect according to whether it is driven by submerged macrophytes or zoobenthos. A combined submerged macrophytes-zoobenthos approach to an aquatic ecosystem will enhance our understanding of the aquatic ecosystem degradation and can function as a guide for the damaged ecosystem restoration practice.
Introduction
Submerged macrophytes and associated zoobenthos communities are two important components in aquatic ecosystems such as shallow lakes, swamps and bogs. Submerged macrophytes such as Hydrilla sp. and Potamogeton sp. are groups of green grass-like photosynthetic organisms that are submerge below the surface of water (Chambers et al., 2007). Zoobenthos refer to the animals that live at the bottom or in the sediment of the water body and are classified into three (3) main groups including microbenthos, example, bacteria and protista, meiobenthos such as Nematode, Turbellaria, Foraminifera, Ostracoda and Copepoda, and macrobenthos such as Mollusca, Polychate, Echinodermata, Crustacea, etc (Herman et al., 1999). Despite the fact of co-existing relationships, the two components have been considered as isolated from one another for a long time. The increasing recognition of the influence of submerged plants or zoobenthos to the environment is significantly increasing which is leading to a rising interest in studying the interaction between submerged macrophytes and zoobenthos. Both submerged plants and zoobenthos are functionally important for the ecosystem as they give direct as well as indirect effects to the biochemistry and physical status of the water ecosystem as a whole (Choudhury et al., 2015; Covich et al., 1999). Well-developed submerged macrophytes vegetation could help to improve the stability and biodiversity of the aquatic ecosystems, thereby increase the ecosystem tolerance to the external pollution load, maintaining lower levels of nutrition and inhibiting cyanobacterial blooms (Sood et. al.,2012), while zoobentho’s ingestion, defecation and bioturbation (including burrows, crawling and foraging) improve the sediment dissolved oxygen (DO) conditions, accelerate the material exchange, promote the decomposition of organic matter, transforming nutrients, enhance the metabolic of pollutants and changing the physical and chemical properties of the sediment (Fisher, 1982; Lee, 2008).
Previous academic researchers have mainly focused on zoobentho’s ecological characters; the role of benthic fauna in the aquatic material circulation and energy flow; the temporal and spatial distribution of the difference of zoobenthos communities and the relationship between zoobenthos and the environmental factors (Hu et. al., 2009; Shan-Nan et. al., 2010). In terms of eutrophication, previous researches were only concerned about the impacts of eutrophication on benthic community structure and diversity as well as zoobenthos as the biological indicator (Kennish, 2016). There are complex interactions between submerged macrophytes and zoobenthos, however, the current studies on the relationship of these two are only in one direction. This paper discusses the recent advances in our understanding of the interaction between submerged macrophytes and zoobenthos. We first outline how submerged macrophytes influence on zoobenthos and vice versa. We then discuss ecological links between submerged macrophytes and zoobenthos. Finally, we explain how the study of submerged macrophytes-zoobenthos interactions may assist our understanding of the consequences of aquatic ecosystem degradation to guide the degraded water-ecosystem restoration.
How Submerged Macrophytes Drive Zoobenthos
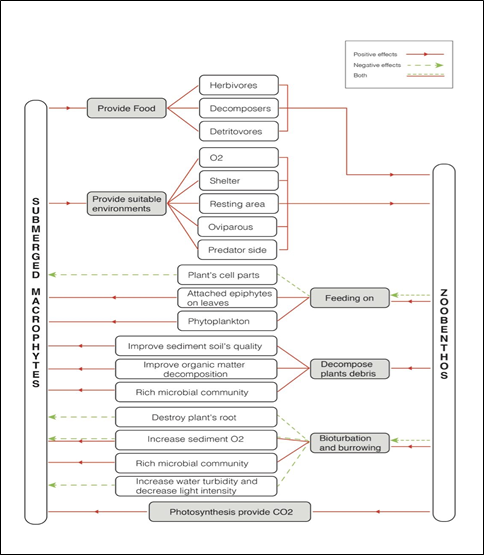
Submerged macrophytes are very important on zoobenthos diversity in aquatic ecosystems as they provide shelter, breeding area as well as site for food (Ali et.al. 2007). Therefore, submerged macrophytes are driving zoobenthos diversity in many ways. One of the main influences of submerged macrophytes on zoobenthos diversification is by providing food both directly and indirectly (Habib & Yousuf, 2015). The food sources of zoobenthos also differ between the following functional groups: herbivores, carnivores, planktivores, omnivores, decomposers, detritivores etc. Herbivores can be further divided into seed predators, browsers, grazers, planktivores etc. Submerged macrophytes can directly provide fresh parts to herbivores, seed predators and browsers, as well as provide decaying plant tissues for decomposers and detritivores. Decaying plant tissues are the primary element for decomposition. Macrophytes have an intermediate length of life cycle which is much longer than that of plankton (Carpenter & Lodge, 1986) which will be served as decaying plant tissues. Sloughing of leaves and stems contribute organic matter and inorganic nutrients needed for decomposition (Engel, 1990). Decomposers and detritivores break down decaying macrophytes and make nutrients available to other tropic levels. In addition, submerged macrophytes also provide attachment surface for epiphytic algae, hence also indirectly supply food for the grazers group within the herbivores (Pettit et.al., 2016). It also provides the attachment site of epiphytes and smaller zoobenthos which are the food for carnivores, planktivores and omnivores zoobenthos (Haiting et.al. 2013). Shamsudin & Sleigh (1995) found that the submerged plant Ranunculus penicillatus has large leave surface for attachment of epiphytes which is almost the average 40% coverage of the stream bed. It is acting as an indirect food supply for the grazers and perhaps gives them protection from predation.
The second drive of submerged macrophytes on zoobenthos is providing the habitat for zoobenthos through increasing DO levels, providing shelter, resting area, spawning site and feeding grounds. An important characteristic of submerged macrophyte beds is that they can alter nutrient cycling and the quality of beds as habitats for zoobenthos such as DO which is the basic need for all animals. (Carraco & Cole, 2002). Submerged plants oxygenate water more efficiently than floating plants (Carpenter & Lodge, 1986). Submerged plants also oxidize their rhizophores and the oxygen produced from photosynthesis will diffuse into the roots and subsequently diffuse into the sediment. The oxidation of sediments supports the microbiological and zoobenthos life process in the sediments. With the increase of the dissolved oxygen in the water, submerged macrophytes “flavour” the environment for zoobenthos. Next, submerged macrophytes also serve as a shelter for zoobenthos from predators, currents and waves. Choudhury et al. (2015) stated that the submerged plants in low water flowing rivers are less branched and less structurally complex which results in lesser surface area for zoobenthos and induce a higher risk of exposure of zoobenthos to predation. In contrast, higher water velocity alters the growth of submerged plants, resulting in more surface area for zoobenthos and better opportunities for zoobenthos to hide from predators as well as functioning as a shelter from water currents and waves. Furthermore, submerged macrophytes provide a site for attachment. Some zoobenthos need attachment sites outside the sediment soil for carrying out life processes such as feeding. Cheruvelil et al. (2002) found that higher zoobenthos densities and biomass are associated with dissected plants rather than undissected plants as there is more surface for attachment, more substrate for foraging and more cover from predators. Submerged macrophytes are also a place for carnivore predation. The high biodiversity found in submerged macrophyte vegetation can supply food for carnivores. Grazers that feed on epiphythic bacteria or algae will create a niche for small carnivores that eat the small herbivores. When large numbers of grazers or herbivores gather for the food available, carnivores may feed directly on herbivores (Likens, 2010a). Last but not least, submerged macrophytes serve as a substratum for oviposition. Submerged macrophytes are used as oviposition sites for invertebrates such as water snails, some cadd and weevil (Maltby et al., 2010). Likens (2010b) stated that Aerial portions of plants provide resting habitats for winged adults during mating and also function as oviposition sites for invertebrates, such as dobson flies (Megaloptera: Corydalidae).
The architecture of submerged macrophyte is one of the factors that affect the submerged macrophyte’s drive on zoobenthos. Firstly, the architecture of submerged macrophyte can directly influence the habitat provided to the zoobenthos. Macrophyte architecture is believed to structure macroinvertebrate communities because the architecture influences the plant surface-to-biomass ratio (Lalonde & Downing 2011). Submerged macrophytes with dissected leaves have a higher association with epiphytic zoobenthos than the undissected ones as dissected structures can provide epiphytic benthic invertebrates with more substrates and more cover from predators (Walker et al., 2013, Bogut et al., 2007; Cheruvelil et al., 2002). Any changes in the macrophyte’s specific composition could indirectly modify the phytophilous zoobenthos community composition and biomass (Cremona et al., 2008). In addition, the architecture of submerged macrophytes can affect the food resources of zoobenthos by influencing the herbivore’s feeding preference. The zoobenthos communities found in different plant species are different (Albertoni et al., 2007; Covich et al., 2004). Some species for example have inhibitory chemicals or hormones which is not only a “flavour” for specific zoobenthos species. Pinowska (2002) mentioned another example in a study in which the subject snails (Galba turricula) consumed more elodea Canadensis than C. Demersum. Therefore, the available E. Canadensis attract more Galba turricula. The structure and architecture of submerged macrophyte also affects the attached epiphyte’s community composition, thus, indirectly affects the feeding of grazers. Hansen et al. (2008) stated that the more delicate leaf structure has been suggested as the main explanation for the higher consumption of periphyton among zoobenthos herbivores which indirectly affected the feeding of grazers. The submerged macrophyte drives zoobenthos by the influence of its architecture through the feeding preferences and the epiphyte’s community composition attachment.
The second factor affecting the submerged macrophyte’s drive on zoobenthos is the nutritional value of the plant tissue. Based on Hansen et al. (2008), plants with high nutrients value are preferred over species with low value in nutrients. Lodge (1991) stated that even though the nutritional value is important for the herbivores’ choice of food, there is generally little difference in the nitrogen content between periphyton and macrophytes. A study conducted by Mattson (1980) and Lodge (1991) also suggested that the nitrogen (protein) as well as other chemical content of vascular macrophytes could limit some herbivory activities. Phenolic content of some macrophytes may be a factor limiting grazing by zoobenthos (Lodge, 1991). Until now, the study on the mechanisms that plants use to defend against herbivory is still lacking. Past studies show that several groups of compounds associated with allelopathy play a part in the communication between plants and other organisms and it is argued that such communication is part of the similarities between plants and animals regarding responses to stress and may contribute to the plant’s defense (Lovett et al., 1989).
How Zoobenthos Drive Submerged Macrophytes
A majority of studies done in the past focused on the general roles that zoobenthos contribute towards the ecosystem, such as being actively involved in the biochemical process of the ecosystem as well as the contribution to the change of the physical environment (Covich et al., 2004; Lindegaard, 1994; Damme et al., 2008). Many zoobenthos species provide essential ecosystem services, such as transport, translocation and transformation of nutrients, such as nitrogen (N) and phosphorus (P) (Vanni, 2002); they provide aeration of sediments via burrowing activities (Palmer, 1997); and control the population of some prey (Cheruvelil et al., 2002). However, the study of the impact of aquatic zoobenthos on the growth of submerged plants has still rarely been a concern. There may be several pathways affecting the submerged plants’ abundance and diversity associated with zoobenthos.
Zoobenthos drive the submerged macrophytes through the feeding process which results in both positive and negative effects on macrophyte. Some zoobenthos directly feed on the macrophyte’s young shoots and other parts such as leaves and branches. Specific species’ feeding preferences will affect the macrophyte’s abundance and coverage, structure and biodiversity. Hilt et al., (2006) stated in his literature where in a German shallow lake, species such as mute swans (Cygnus olor), coot (Fulica atra), rudd (Scardinius erythrophthalmus) and grass carp (Ctenopharyngodon idella) are mainly known to feed on submerged macrophytes and to affect the macrophyte’s abundance, coverage structure and biodiversity over time. Zoobenthos scraping off the attached algae on the submerged macrophyte’s surface is conducive to the growth of submerged macrophytes. Bronmark (1985) supported the statement where grazing by epiphyte grazers zoobenthos promote the growth rate of the submerged macrophyte host. The grazing epifauna scrape the submerged macrophyte’s leaf surface for food. They remove both organic debris and live algal epiphytes from macrophyte’s leaf effectively. The macrophyte surface in grazer –excluded treatment shows a thicker and highly opaque later of epiphytes cover on the leaf surface. It restricted the nutrient exchange of the macrophyte and shows decreased productivity compared to treatments with grazers (Howard & Short, 1971). Thus, it is suggested that zoobenthos scraping is giving a positive effect to the growth of submerged macrophytes and may also prolong the lifespan of macrophytes by reducing the conditioning through necrotrophic bacteria (Hootsmans & Vermaat, 1985). The extended lifespan of macrophytes is due to the fact that the grazing of the epiphytic zoobenthos community has reduced the rate of development of harmful bacteria (Rogen & Breen, 1983).
Secondly, zoobenthos drive the submerged macrophytes by accelerating the detritus decomposition. The sludge residue on the sediment was cleared and the permeability of sediment was improved. Fukuhara et al. (1987) stated that zoobenthos can perform sludge reduction through their ingestion and excretion. The detritus decomposition process done by zoobenthos can also enhances the organic matter production. A study done by Canuel et al. (2007) showed that the structure of the epibenthic animal community strongly influenced the abundance and quality of organic matter accumulated in seagrass sediments. Benthic ecosystems play an important role in the storage and cycling of organic matter and nutrients (Canuel et al., 2007). Zoobenthos process the organic matter produced from the submerged plants’ decay directly and indirectly. The direct processing mode includes the use or uptake of organic matter through benthic fauna via ingestion and is used for metabolic growth or released back to the ecosystem through excretion. It is indirectly referring to the repackage or fragment organic matter into smaller particles without ingesting them while fragmenting detritus into fine particles to facilitate the ingestion of other microorganisms (Palmer, 1997; Prather et al., 2013). This can also maintain the microorganism’s biodiversity in the sediment which can improve the quality of the soil.
Another method is that zoobenthos drive the submerged macrophyte through bioturbation. Bioturbation can increase the nutrient availability. Literature by Prather et al. (2013) shows that zoobenthos can redistribute and alter nutrient availability within an ecosystem through the consumption and egestion of plants and detritus, and by physically moving materials and disturbing sediments via bioturbation and bioerosion. It can also lead to increased rates of nitrification of excreted ammonium, increased nitrate flux to anoxic sediment layers, precipitation of excreted phosphorus and the subsequently increase of denitrification rates (Steiner & Roy, 2003). Bioturbation of zoobenthos oxidize the sediment using ventilation. The ventilation mediates increase in transport of dissolved oxygen and N-compounds as well as oxygenates near-surface pore waters by increasing diffusion of oxygen from the overlying water (Kristensen et al., 1995; Pelegri & Blackburn, 1995; Hansen & Kristensen, 1998). Besides, bioturbation also gives indirect effects on the nutrient cycle by redistributing organic matter deposited on the sediment surface by sediment reworking (Zhong et al., 2008). Burrowing zoobenthos mostly consume and translocate nutrients into the water column for the uptake for submerged macrophytes. This will alter the physical environment and increase the exchange of nutrients between water and sediment. Sometimes translocation happens simultaneously accompanied by a transformation of nutrients from one chemical form to another (Covich et al., 1999; Vanni 2002). However, the burrowing and feeding process will sometimes destroy plants’ roots and negatively affect the macrophyte’s growth. Few zoobenthos such as crayfish, plant–parasitic nematodes and some aquatic insects eat, destroy and strongly affect the macrophyte’s biomass and species’ composition (Strayer, 2009). Furthermore, zoobenthos drive the photosynthesis process of submerged macrophyte. The respiration of zoobenthos releases carbon dioxide (CO2) which is necessary to submerged plants to complete the photosynthesis process. It is one of the main organic carbon resources for the aquatic environment (Palmer, 1997). Some submerged plants take the provided carbon dioxide in and release some organic carbon into water or precipitate as carbonate salts. This release contributes to the metabolism of bacteria, epiphytic microorganisms as well as zoobenthos (Carpenter & Lodge, 1986).
Submerged Macrophytes and Zoobenthos – Linked or not?
There are strong linear and non-linear two way interactions between submerged macrophytes and zoobenthos (Vanni, 2002). The two components are linked by the material recycle and energy flow of aquatic ecosystems. They are mainly reflected by the top-down effect and down-top effect in the water ecosystem. The submerged macrophytes on zoobenthos down-top effect is a concept, which was first suggested by Murdoch (1966). The concept is that the density and biomass of the lower trophic level plays a decisive role in the population structure of the higher trophic level species while the top-down effect is briefly described by Hairston et al. (1960). The population structure such as abundance, biomass, and biodiversity in the lower trophic level species is supposedly controlled by the higher trophic level species through process such as predation. Studies have shown that zoobenthos have a variety of ways to achieve top-down effects.
The down-top effect of submerged macrophytes on benthic fauna is achieved by the following approaches. Firstly, lower trophic level submerged macrophytes provide food. The submerged macrophytes influence zoobenthos through leaf morphology, nutrient content, and specific chemical substances, providing direct food supply and indirect feeding facilities. This finding is linear with Bolser et al. (1998) and Cronin et al. (2002) who have demonstrated the selective feeding of animals that has been attributed to the physical, chemical and nutritious properties of the macrophytes. As producers, the submerged macrophytes’ changes will affect higher trophic levels (Ware & Thompson 2005). The availability of producers is then said to influence the number of zoobenthos through their role as a source of food, followed by providing habitat. Submerged macrophytes provide a physically and chemically complex habitat in aquatic ecosystems and its architectural features of this habitat can affect the zoobenthos species’ diversity, density and distribution (Bogut et al., 2007; Carpenter & Lodge, 1986). The role of submerged macrophytes in providing habitat affects the survival of the zoobenthos itself. Thus, this explained the down-top effect of submerged macrophytes on benthic fauna.
The top-down effect, creating predator pressure, is one of the ways in which zoobenthos affect the submerged macrophytes. Fox et al. (2010) stated that the top-down effect can alter biomass, species composition, and nutritional quality of producers which in this case are the submerged macrophytes. The community composition of submerged macrophytes can be controlled by ingesting specific species of submerged macrophytes. As suggested by Sheldon (1987), the selective feeding of Physa to 14 submerged macrophytes suggests that spirals tend to select those submerged macrophytes that grow faster. Wang et al. (2006) demonstrated that excessive stocking of crabs resulted in a decrease of submerged macrophytes biomass. Wang & Zhao (1999) & Xu et al. (2003) also show that the successive over-stocking of the crab leads to the deterioration of water quality and the exhaustion of natural food resources which resulted in declines of crab sizes and yields. Fox et al. (2010) suggested that predator pressure could alter the population structure of submerged macrophytes as predators may create “cascading” top-down effects by reducing grazer abundance. This is also accomplished through indirectly increasing primary producer biomass by releasing them from grazing pressure. As an example for this case the presence of predatory fish and crabs is used which can initiate a trophic cascade by reducing or inhibiting invertebrate grazers, which, in turn, allows algal biomass to accumulate (Duffy et al. 2005; Moksnes et al. 2008; Baden et al. 2010).
Also, the zoobenthos can enhance the growth and survival submerged macrophytes. Bivalves in benthos and other species can filter phytoplankton, aquatic plants and phytoplankton. For example, suspension-feeding bivalves can serve to improve water quality in eutrophic estuaries by exerting top-down control on phytoplankton populations. Zoobenthos such as gastropods can scrape off submerged macrophytes surface attachment organisms, reduce the rate of development of necrotrophic bacteria which is a treat to the growth of submerged macrophytes (Hootsmans & Vermaat, 1985). It can also help in expanding the lifespan of macrophytes for at least 58% accordingly to the experiment done by Rogers and Breen (1983). Lodge and Kelly (1985) demonstrated that gastropods such as snail population size depend on the macrophyte abundance where a severe decrease of macrophyte richness could lead to the decrease of the Lymnaeastagnalis population to up to 99%, and the population of Bithynia tentaculate to up to 35%. Aquatic plants such as submerged plants have a mutual relationship with snails. Underwood (1991) shows that there is a significant increase in growth of Ceratophyllum sp. in terms of longer and healthy nodes of leaves and more growing tips with the presence of snails or in water chemically conditioned by snails. Furthermore, zoobenthos help to create a suitable habitat for the lower trophic level submerged macrophytes such as improving the physical and chemical properties of the sediment. For example, bioturbation and the burrowing process of zoobenthos can physically redistribute the materials and disturb sediments. This can alter nutrient availability within an ecosystem, increase the rates of nitrification of excreted ammonium, nitrate flux to anoxic sediment layers, precipitation of excreted phosphorus and results in a subsequent increase of denitrification rates (Steiner & Roy, 2003). In addition, some zoobenthos excrete nitrogen and phosphorus in form of ammonium and phosphate which are needed by the growth of plants (Carpenter & Lodge, 1986).
Implications for the Aquatic Ecosystem Degradation and Restoration
As the global population increases, the surface water demand has increased drastically around the world (Khan et al., 2014). Due to the shortage of clean water supply, degradation of aquatic ecosystems has become apparent to the public. The cumulative studies related to aquatic ecosystem remediation has significantly risen in the past five decades. Due to the important roles of submerged macrophytes, the rehabilitation and reconstruction of submerged macrophytes community had become a main objective for eutrophication management. So far, many Chinese researchers had done some successful studies on the submerged macrophytes communities’s effects on eutrophic water bodies restoration. Related researches focused on the relevant factors and effects of submerged macrophytes such as the effects of light intensity (Lisha et al., 2013), the change of nutrient level and other water quality parameters (Chen et al., 2013; Dai et al., 2015), its purification effects on the restoration (Le et al., 2010; He et al., 2015), its effects on nitrogen cycling bacteria and other related mechanisms (Wang et al., 2013; Han et al., 2016). As the degradation of submerged macrophytes and aquatic animals is an important feature of aquatic ecosystem degradation. Recent research started to shift toward the combination effect of submerged macrophytes and aquatic animals on aquatic remediation (Gao et al., 2017). However, the combination interactions and effects of submerged macrophytes and aquatic zoobenthos to the recovery are still rarely understood.
From the viewpoint of the interactions between submerged macrophytes and zoobenthos, the degradation mechanism of aquatic ecosystems can be divided into two types. One is the degradation of aquatic ecosystems driven by bottom-up effects such as water eutrophication that caused the submerged macrophytes community degradation. This led to changes in the zoobenthos’ community structures as different submerged plants within an ecosystem create various microhabitats which should result in different assemblages of zoobenthos (Hann, 1995). Once the habitat is disturbed, the zoobenthos community plays an important role in mediating both physical and chemical processes near the sediment-water interface by enhancing the suspension and transportation of sediments and encouraging a material exchange (Chen et al., 2015) for biochemical reactions through their burrowing, feeding, locomotive, respiratory and excretory activities (Fisher, 1982), which are also degraded. Another example for the bottom-up degradation effect is the over-stocking of grass carp that feed on submerged macrophytes, leading to the disappearance of submerged macrophytes communities which eventually results in the disappearance of adhesive zoobenthos (Lynch, 2009). Some bottom-up degradation happen as a result as the biological invasion of the submerged macrophytes. The change in structure and species abundance of macrophytes community will cause the shift of the zoobenthos community(Cai et al., 2015). This might alter the ecosystem’s processes such as the hydrology and water quality and thereby changes the role of existence for all species (Vitousek et al., 1997). In the research conducted by Eiswerth & Johnson (2000), the Eurasian watermillfoil Myriophyllum spicatum has proven can reduce the water quality in its invasive range (increased nutrient loading, reduced dissolved oxygen and changes in water temperature), which in turn decreased the numbers and cover of native plant species and caused negative effects to fishes and other animals such as zoobenthos. This will eventually lead to the degradation of the aquatic ecosystem.
The top-down factors of the degradation of water ecosystems are for example the excessive zoobenthos feeding and destruction of root systems that lead to degradation of submerged macrophyte communities. Strayer (2009) stated that some zoobenthos species destroy or feed on the roots or other parts of the submerged plants, causing a drop in macrophyte biomass and species composition. This is the reason why zoobenthos have been used for biological control of nuisance weeds such as milfoil. On the other hand, the closed linked relationship between submerged macrophytes and zoobenthos also provides a revelation water remediation effect for the restoration of degraded ecosystems. Targeted recovery strategies are applied according to the different mechanisms of aquatic ecosystem degradation. This is due to the fact that submerged macrophytes and zoobenthos are the driving factors of the degradation in aquatic ecosystems of the down-top effect, as well as the top-down effect mechanism respectively. The restoration strategy for the degradation of the aquatic ecosystem driven by a down-top effect should focus on submerged macrophytes, eliminate the factors that cause the changes of submerged macrophyte communities, and restore and rebuild the original submerged macrophytes’ community. Followed by the response to the degradation of the aquatic ecosystem driven by the top-down effect, the restoration strategy should start with benthic animals and restore as well as reconstruct the original benthic communities. For instance, Muotka T. ET. Al (2002) suggested adding large woody debris to increase retention potential which can help in speeding up the benthic community restoration by yielding a healthier ecosystem with natural-like food webs and trophic dynamics.
Conclusion
We can conclude that submerged macrophytes and zoobenthos assemblages are ecologically important components of freshwater ecosystems. The ecological integrity and health of aquatic systems is depending on the health of submerged macrophytes and zoobenthos communities. The interaction between them is bilateral where submerged macrophytes provide food for herbivores, decomposers and detritivores zoobenthos; provide a suitable habitat by providing oxygen, shelter, resting area, site for oviparous and site for predation while zoobenthos provide carbon dioxide to promote photosynthesis and improve the sediment soil’s quality for flavouring macrophyte growth.
Submerged macrophytes and zoobenthos are closely linked and the interaction gives positive effects on maintaining the balance and health of the aquatic ecosystem. The mechanisms of interaction between submerged macrophytes and zoobenthos are concluded by dividing them into two types: the bottom-up effect (the submerged macrophytes’ influences on zoobenthos) and the top-down effect (zoobenthos’ influences on submerged macrophytes). The down-top effect refers to food resources availability and habitat environment while the top-down effects include the predator pressure, improving competition, and also improving the habitat environment of submerged macropyhtes. Hence, it is very important to focus on these two subjects during the implications for aquatic ecosystem degradation studies and restoration projects. The drives of aquatic ecosystem degradation can also be classified into bottom-up or top-down, as well as different mechanisms or strategies that should be applied accordingly to different types of drives. For the degradation of the aquatic ecosystem driven by down-top effects, the remediation strategy should focus on the submerged macrophytes’ recovery while the restoration strategy should begin with the restoration of the zoobenthos’ communities in case of a top-down effect.
Images and Tables